Biopharma, Medical Devices, CRO’s need to comply with growing demands of confidential data disclosures under regulations like EMA Policy 0070, EMA (EU) CTR 536/2014, Health Canada PRCI, others. This necessitates a paradigm shift for Medical Writing / Document generation, by incorporation ‘Security by Design’ or ‘Proactive Authoring’ principles. Till this shift happens, Pharma organizations need to adopt an Enterprise level, AI powered, Data & Document Anonymization Solution which can cater to protecting sensitive PII, PHI, CCI data for clinical trials disclosures, voluntary data sharing agreements across all Therapeutic Areas and business functions.
Industry Challenges
Pharma organizations employ manual or semi-automated methods to manage sensitive information in documents. Manual redaction is time consuming, often error prone and highly inefficient. As regulations, document volumes and sensitive data variables grow, pharma organizations are bound to face scalability challenges. Some of the key challenges faced today are –
- Under Redaction – Sensitive data can remain un redacted due to the manual process, work fatigue or document complexity. Often, there are cases of inefficient redaction, where certain data elements can be reidentified.
- Over Redaction – Too many / blanket redactions diminish the utility of disclosed data. Health Regulators will often push back documents which seem overreacted
- Multi language – Document reviewers are often limited to the knowledge of languages known to them
- Scalability - The process is not scalable to support all enterprise level data anonymization business use cases involving data, documents, images, audio, video.
- Bias – There is a high risk of bias based on reviewer’s subjective knowledge and decision making
- Workflows – In efficient, manual workflows, including approval and auditing workflows.
These challenges can lead to strict penalties for improper data handling, while data breaches can badly damage an organization’s reputation and revenue.
eRAIS solution for the Pharma Industry
In Part 1 of our blog, we touched upon the existing marketplace for Document Anonymization Solutions. We also presented our point of view on the Data Anonymization requirements for an enterprise solution for Life Sciences organizations.
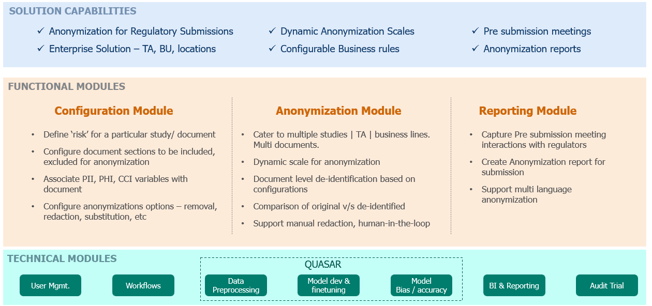
Based on these findings and industry needs Coforge has developed Gen AI powered eRAIS (electronic Redaction and Anonymization Intelligent System) powered by our Quasar Document AI framework to address the unmet needs of the pharma Industry. Some of salient features of the solution are –
- Built on our proven Quasar Responsible AI framework
- Deployment flexibility – SaaS, On Premise, Hybrid
- Configurable – easy to configure business rules & risk across documents, sections, paragraphs, lines, context, others
- Support for images, text, audio
- Multi language support
- Customized / Pre trained on CCI variable definitions & data
Benefits
eRAIS is powered by Coforge Quasar AI, which is our proprietary and proven AI framework for all things AI. The advanced Gen AI capabilities of Quasar enable contextual redaction of sensitive data with easy abilities to support a growing list of company confidential information.
Some of the key benefits of eRAIS include:
- Automation – Automated Redaction powered by configured business rules along with Human-in-the-Loop workflows
- Accuracy – Empowered by highly accurate AI models with continuous learning & improvement capabilities
- Scalable – Supports real time v/s batch mode, multi language, as well as multiple input formats like text, images, audio, others.
- Configurable – Ease of setting up business rules across Organization, Geography, Therapeutic Area, Study specific levels
- Efficiency – Improved Operational efficiency through
- Easy to set up business rulesb
- Dynamic risk scaling
- Original v/s Redaction comparison
- Reduced hand offs for approval
- In built Auditing
- Integration – Capabilities to integrate with leading content management and document management systems
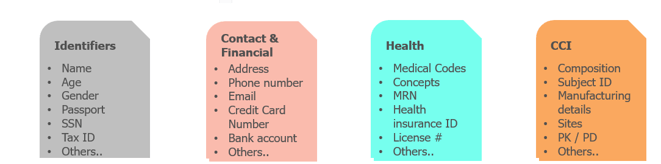
- Coverage – More than 50+ pre-configured sensitive data elements/variables
Connect with us to learn more about how Quasar eRAIS can help with your enterprise document anonymization / redaction needs and how Quasar AI can help drive broader AI CoE and use case acceleration across your enterprise.







